All solid-state batteries are attracting attention as a powerful candidate for a new generation of batteries because they replace traditional organic electrolytes with solid electrolytes, which are expected to improve safety and extend service life. Recently, a solid electrolyte which can further improve the performance has been discovered.
This article refers to the address: http://

Development sample
This is one of the sulfide-based solid electrolytes - Li10GeP2S12. The ionic conductivity indicating the lithium diffusion rate is extremely high, and can reach 1.2×10-2 S/cm at normal temperature (27 ° C). It was developed by a research team at the Tokyo Institute of Technology, Toyota Motor, and the High Energy Accelerator Research Institute. In the words of Takino, a professor of physical and electronic chemistry at the Graduate School of Integrated Science and Engineering of the Tokyo Institute of Technology, the leading research and development program, it is "breaking the obstacle of 10-2 S/cm at room temperature that solid electrolytes could not achieve".
Refresh the record that has been maintained for 30 years
As an important issue in the practical process of all-solid batteries, there has been a problem that the solid electrolyte ion conductivity is low. One of the evidences is that the highest known Li3N (ion conductivity 6×10-3S/cm at room temperature) has not been improved by an order of magnitude over the past 30 years since it was discovered in the 1970s.
The material discovered this time reached 1.2×10-2S/cm at room temperature, achieving the same ionic conductivity as the existing mainstream organic electrolyte (Fig. 1). Moreover, the ionic conductivity superior to the organic electrolyte is shown at a low temperature.
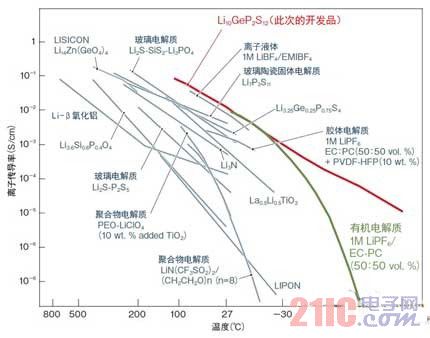
Figure 1: ION conductivity is better than electrolyte
The solid electrolyte developed by Tokyo Institute of Technology and Toyota Motor has a very high conductivity of 1.2×10-2 S/cm at room temperature. It has characteristics over conventional lithium ion conductors such as organic electrolytes and polymer electrolytes currently in use. (picture is based on data from Tokyo Institute of Technology)
Further, since only the lithium ions move in the solid electrolyte of the all-solid battery to carry all the current, the number of migration is 1. In the electrolyte, not only the cation, lithium ion, but also the anion also moves, so the migration number is low. Due to this feature, the solid electrolyte developed this time is considered to exhibit superior performance over organic electrolytes.
The results of this work were discovered through "repeated exploration of sulfide-like substances that may have high ionic conductivity" (Wilderness). "After finding the candidate material, it took about a year to work on the single-phase synthesis process." (è…野)
Detecting the distribution of structure and lithium
In addition to increasing ionic conductivity, another major achievement of this study was the analysis of the Li10GeP2S12 structure. The neutron diffraction measurement of the ultra-high resolution powder neutron diffraction device "SuperHRPD (BL08)" in the high-intensity proton accelerator facility "J-PARC" finally determined the crystal structure.
As a result of the analysis, it was found that Li10GeP2S12 has a structure different from that of the previous solid electrolyte (Fig. 2). Specifically, Li10GeP2S12 is a three-dimensional skeleton structure material, and lithium has a high lithium conductivity in the skeleton structure because lithium exists in a chain structure. At the same time, it was also found that the proportion of lithium in the constituent materials was high, which confirmed the reason for the increase in ionic conductivity.
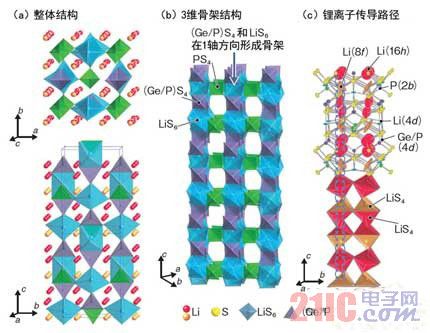
Figure 2: Lithium inside the skeleton structure exists in a chain
The solid electrolyte (Li10GeP2S12) developed this time is different from the former solid electrolyte and is a substance having a three-dimensional structure. (c) The upper part of the figure is a thermal vibration of lithium ions, and lithium ions vibrate vigorously in the up and down direction (c-axis direction) and affect ion conduction. (picture is based on data from Tokyo Institute of Technology)
Finding materials with the same structure as this new material is expected to synthesize materials with higher ionic conductivity.
The research team has also strengthened its practical efforts while exploring materials. Toyota has prototyped an all-solid battery using the Li10GeP2S12 (Figure 3). Lithium cobaltate (LiCoO 2 ) and indium (In) were used for the positive and negative electrode materials, respectively, and the charge and discharge characteristics were measured.
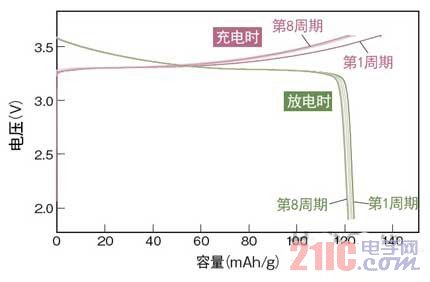
Figure 3 Stable charge and discharge cycle characteristics
Toyota Motor has prototyped an all-solid battery using Li10GeP2S12 as a solid electrolyte. It has been confirmed that the capacity exceeds 120 mAh/g, and the performance does not deteriorate even after repeated charge and discharge for about 10 times. (picture is based on data from Tokyo Institute of Technology)
As a result, a very stable charge and discharge curve was obtained. Specifically, when the current density was 14 mA/g, a discharge capacity exceeding 120 mAh/g was exhibited. After the second cycle, about 100% of the charge and discharge efficiency was exhibited, and it was confirmed that the charge and discharge were stable up to the eighth cycle.
It is said that in the future, practical issues such as the long-term stability of solid electrolytes and the optimal combination of positive and negative electrodes will be solved one by one.
Furniture Lights,Furniture Lighting,Furniture Cabinet Lights,Furniture Bedroom Lighting
Dongguan baiyou electronic co.,ltd , https://www.dgbaiyou.com